Are Water Changes Actually Necessary?
- Thread starter kazvorpal
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
- Status
- Not open for further replies.
OgreMkV
Father of Earth's Next Emperor
OK, the OP has requested the scientific evidence of the requirement for water changes. In this article I will present the information that I have found to date. It was actually fairly difficult mainly because this is such a well known requirement that all the research was done well before the 1980s. However, while I feel that I can be trusted to present the scientific information correctly, anyone is free to do the work that I have and verify what I'm saying.
BTW: The chemical reactions look like crap in the forum text... sorry. If a number is in front of letters, it is regular, if it is after some letter, it should be subscripted.
First a quick lesson in the Nitrogen cycle.
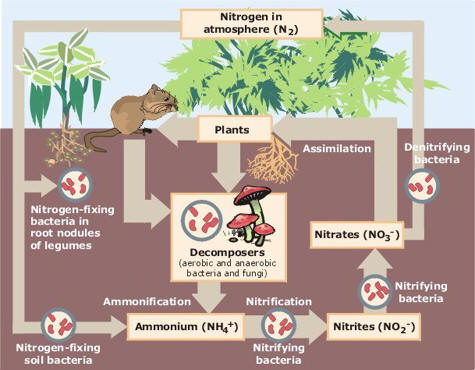
Source: http://www.epa.gov/maia/html/nitrogen.html
Now, this shows land, but where the cycle is slightly different for fresh or saltwater, I'll note the changes.
We'll start with the fish, since this is mainly what the conversation is about. Fish need nitrogen for literally everything in their bodies. Nitrogen is a primary component of every protein and strand of genetic material (DNA and RNA) in every cell of every organism on the planet. However, there is plenty of nitrogen available and organisms (including fish) have no problem finding sufficient nitrogen in easily usable forms. There's generally some nitrogen left over and (in land animals) that is excreted in the form of uric acid (urea AKA pee-pee).
However, fish do something very different, they excrete an amide called glutamine from their gills. The glutamine is hydrolized in water to produce glutamic acid and ammonia. ( http://www.elmhurst.edu/~chm/vchembook/633ureacycle.html).
The appropriate reactions are (and enthalpies for the curious or insane):
[FONT=Courier, monospace]Glutamine (aq.) + [/FONT][FONT=Courier, monospace]H20 [/FONT][FONT=Courier, monospace]-* [/FONT][FONT=Courier, monospace]glutamate+ [/FONT][FONT=Courier, monospace]+ [/FONT][FONT=Courier, monospace]NH4+,[/FONT]
[FONT=Courier, monospace]AH= -5160±70cal.[/FONT]
[FONT=Courier, monospace]Glutamate' + H+ -+ glutamic acid,[/FONT]
[FONT=Courier, monospace]AH[/FONT][FONT=Courier, monospace]= [/FONT][FONT=Courier, monospace]-950[/FONT][FONT=Courier, monospace]± [/FONT][FONT=Courier, monospace]30 cal.[/FONT]
(http://www.biochemj.org/bj/071/0395/0710395.pdf)
Now for the not so chemically literate that means that asglutamine is ejected from fish, it reacts with water to form glutamate ion and ammonium ion. The glutamate then reacts with hydrogen ions for form glutamic acid.
Other bacteria (called decomposers) also contribute to the nitrogen load of the aquarium. Uneaten food, fish poop, decaying plants, heck, even fish scales, are all consumed (slowly) by bacteria. This process releases ammonia.
Note for freshwater aquariests: It is true that plants will uptake ammonia, nitrates, and nitrites. This paper gives a lengthy discussion of the topic (http://www.hort.purdue.edu/rhodcv/hort640c/nuptake/nu00001.htm)
However, there is some blockage of the uptake of one form of nitrogen for others (some plants like ammonia, while some prefer nitrites). Also, the pH is an important moderator for the uptake of these nutrients into plants. The following discussion will note that the change of one form of nitrogen to another produces an acidic environment.
Here's where the bacteria get started (see chart above). First bacteria that nitrify ammonium for their energy source (generally of Nitrosomonas or Nitrosococcus genus (at least for aquariums)). Do the following:
NH3 + O2 + 2H+ + 2e− → NH2OH + H2O
NH2OH + H2O → NO−2 + 5H+ + 4e−
What this means it that the bacteria use a small amount of energy (2 electrons) and two hydrogen ions to create ammonium hydroxide and water. Then they convert the ammonium hydroxide into nitrites, 5 hydrogen ions, and more energy (4 electrons). It's not very efficient, but it's enough for these bacteria to live on. Note that there are 5 hydrogen ions produced for every 2 ions used... this increases the acidity of the water.
Now the nitrifying bacteria that work on nitrites go to work. These are generally Nitrobacter (freshwater and marine) or three other genuses in marine environments. They do the following:
NO−2 + H2O → NO−3 + 2H+ + 2e−
Basically, the is taking the nitrites and hydrolysing it to form nitrates, 2 more hydrogen ions and 2 electrons for the energy. Again, more hydrogen ions making the water more acidic.
This is where the nitrogen cycle in freshwater takes generally stops. These reactions take place in the presence of oxygen. Freshwater aquariests rarely go any further with this because the equipment necessary to create an anaerobic environment for the denitrifying bacteria is rare, expensive, homemade, or not needed because water changes are easier to do.
For the marine types, we'll take it one step further. The bacterial species Thiobacillus denitrificans, Micrococcus denitrificans and the genus Pseudomonas use oxygen as a terminal electron receptor which is why it must be in an anaerobic environment. Which, in marine tanks, is generally deep in the pores of the live rock and deep under the sand (lower than about 3-4 inches) which is why a 6 inch sand bed is recommended. Note that any flow to an area renders this chemical reaction impossible so there are no filters that can allow nitrifying bacteria to grow and flourish. The chemical reaction in these bacteria is as follows:
2NO3- + 10e- + 12H+ → N2 + 6H2O
Note the use of large numbers of hydrogen ions in this reaction. However, rememeber the above reactions, this only removes the hydrogen ions generated from the nitrite to nitrate step.
Also, something to keep in mind, cyanobacteria (often wrongly called an algae) does the reverse of this and converts gaseous nitrogen back into ammonium or one of the nitrogen species for use by itself and/or plants or true algaes. So if you have a cyano outbreak, all the nitrifying and denitrification bacteria in your tank are just keeping up.
This paper is critical for our discussion: https://kb.osu.edu/dspace/bitstream/1811/36344/1/OH_WRC_490.pdf
It describes the toxicity of various nitrogen products to the common guppy (Poecilia reticulus). {NOTE: This paper also describe the problems the researchers had in keping the ammonia levels constant in the tanks as evaporation of the water caused the ammonia concentration to increase by 5% over four days. The tests also observed a change in pH from 7.5 to 6.9 in four days.}
The final results are that 1.26mg of ammonia per liter has a LD50. That's lethal dose 50%... or the level at which 50% of the fish will die. 199 mg/L of nitrate will do the same thing. That's within a three day time frame.
When ammonia and nitrate are both present, LD50 occurs when the the concentrate of ammonia is .5mg/L and nitrate is 30mg/L at the same time.
Just because most Americans aren't familiar with the metric system... a grain of rice weighs between 20-30 milligrams. A ten gallon tank is roughly 30 liters.
What does this mean for the aquariest?
I hope that at least as far as nitrogen compounds are concerned, that nature isn't present in our tanks and that it is difficult to recreate the necessary conditions in our tanks. Keep in mind that many of you (like myself) really like Amazon species. The amazon has a flow rate of 7 million cubic feet (that's 52 million gallons) of water per SECOND. That's why they don't have any trouble with nitrogen build up.
So, I hope that water changes are a part of your tank regime.
With a little luck and more spare time, I'll do some research on the other components of the tank (buffer/pH chemistry, dissolved oxygen, TDS, etc) and come up with some more... but this is long enough already.

BTW: The chemical reactions look like crap in the forum text... sorry. If a number is in front of letters, it is regular, if it is after some letter, it should be subscripted.
First a quick lesson in the Nitrogen cycle.

Source: http://www.epa.gov/maia/html/nitrogen.html
Now, this shows land, but where the cycle is slightly different for fresh or saltwater, I'll note the changes.
We'll start with the fish, since this is mainly what the conversation is about. Fish need nitrogen for literally everything in their bodies. Nitrogen is a primary component of every protein and strand of genetic material (DNA and RNA) in every cell of every organism on the planet. However, there is plenty of nitrogen available and organisms (including fish) have no problem finding sufficient nitrogen in easily usable forms. There's generally some nitrogen left over and (in land animals) that is excreted in the form of uric acid (urea AKA pee-pee).
However, fish do something very different, they excrete an amide called glutamine from their gills. The glutamine is hydrolized in water to produce glutamic acid and ammonia. ( http://www.elmhurst.edu/~chm/vchembook/633ureacycle.html).
The appropriate reactions are (and enthalpies for the curious or insane):
[FONT=Courier, monospace]Glutamine (aq.) + [/FONT][FONT=Courier, monospace]H20 [/FONT][FONT=Courier, monospace]-* [/FONT][FONT=Courier, monospace]glutamate+ [/FONT][FONT=Courier, monospace]+ [/FONT][FONT=Courier, monospace]NH4+,[/FONT]
[FONT=Courier, monospace]AH= -5160±70cal.[/FONT]
[FONT=Courier, monospace]Glutamate' + H+ -+ glutamic acid,[/FONT]
[FONT=Courier, monospace]AH[/FONT][FONT=Courier, monospace]= [/FONT][FONT=Courier, monospace]-950[/FONT][FONT=Courier, monospace]± [/FONT][FONT=Courier, monospace]30 cal.[/FONT]
(http://www.biochemj.org/bj/071/0395/0710395.pdf)
Now for the not so chemically literate that means that asglutamine is ejected from fish, it reacts with water to form glutamate ion and ammonium ion. The glutamate then reacts with hydrogen ions for form glutamic acid.
Other bacteria (called decomposers) also contribute to the nitrogen load of the aquarium. Uneaten food, fish poop, decaying plants, heck, even fish scales, are all consumed (slowly) by bacteria. This process releases ammonia.
Note for freshwater aquariests: It is true that plants will uptake ammonia, nitrates, and nitrites. This paper gives a lengthy discussion of the topic (http://www.hort.purdue.edu/rhodcv/hort640c/nuptake/nu00001.htm)
However, there is some blockage of the uptake of one form of nitrogen for others (some plants like ammonia, while some prefer nitrites). Also, the pH is an important moderator for the uptake of these nutrients into plants. The following discussion will note that the change of one form of nitrogen to another produces an acidic environment.
Here's where the bacteria get started (see chart above). First bacteria that nitrify ammonium for their energy source (generally of Nitrosomonas or Nitrosococcus genus (at least for aquariums)). Do the following:
NH3 + O2 + 2H+ + 2e− → NH2OH + H2O
NH2OH + H2O → NO−2 + 5H+ + 4e−
What this means it that the bacteria use a small amount of energy (2 electrons) and two hydrogen ions to create ammonium hydroxide and water. Then they convert the ammonium hydroxide into nitrites, 5 hydrogen ions, and more energy (4 electrons). It's not very efficient, but it's enough for these bacteria to live on. Note that there are 5 hydrogen ions produced for every 2 ions used... this increases the acidity of the water.
Now the nitrifying bacteria that work on nitrites go to work. These are generally Nitrobacter (freshwater and marine) or three other genuses in marine environments. They do the following:
NO−2 + H2O → NO−3 + 2H+ + 2e−
Basically, the is taking the nitrites and hydrolysing it to form nitrates, 2 more hydrogen ions and 2 electrons for the energy. Again, more hydrogen ions making the water more acidic.
This is where the nitrogen cycle in freshwater takes generally stops. These reactions take place in the presence of oxygen. Freshwater aquariests rarely go any further with this because the equipment necessary to create an anaerobic environment for the denitrifying bacteria is rare, expensive, homemade, or not needed because water changes are easier to do.
For the marine types, we'll take it one step further. The bacterial species Thiobacillus denitrificans, Micrococcus denitrificans and the genus Pseudomonas use oxygen as a terminal electron receptor which is why it must be in an anaerobic environment. Which, in marine tanks, is generally deep in the pores of the live rock and deep under the sand (lower than about 3-4 inches) which is why a 6 inch sand bed is recommended. Note that any flow to an area renders this chemical reaction impossible so there are no filters that can allow nitrifying bacteria to grow and flourish. The chemical reaction in these bacteria is as follows:
2NO3- + 10e- + 12H+ → N2 + 6H2O
Note the use of large numbers of hydrogen ions in this reaction. However, rememeber the above reactions, this only removes the hydrogen ions generated from the nitrite to nitrate step.
Also, something to keep in mind, cyanobacteria (often wrongly called an algae) does the reverse of this and converts gaseous nitrogen back into ammonium or one of the nitrogen species for use by itself and/or plants or true algaes. So if you have a cyano outbreak, all the nitrifying and denitrification bacteria in your tank are just keeping up.
This paper is critical for our discussion: https://kb.osu.edu/dspace/bitstream/1811/36344/1/OH_WRC_490.pdf
It describes the toxicity of various nitrogen products to the common guppy (Poecilia reticulus). {NOTE: This paper also describe the problems the researchers had in keping the ammonia levels constant in the tanks as evaporation of the water caused the ammonia concentration to increase by 5% over four days. The tests also observed a change in pH from 7.5 to 6.9 in four days.}
The final results are that 1.26mg of ammonia per liter has a LD50. That's lethal dose 50%... or the level at which 50% of the fish will die. 199 mg/L of nitrate will do the same thing. That's within a three day time frame.
When ammonia and nitrate are both present, LD50 occurs when the the concentrate of ammonia is .5mg/L and nitrate is 30mg/L at the same time.
Just because most Americans aren't familiar with the metric system... a grain of rice weighs between 20-30 milligrams. A ten gallon tank is roughly 30 liters.
What does this mean for the aquariest?
I hope that at least as far as nitrogen compounds are concerned, that nature isn't present in our tanks and that it is difficult to recreate the necessary conditions in our tanks. Keep in mind that many of you (like myself) really like Amazon species. The amazon has a flow rate of 7 million cubic feet (that's 52 million gallons) of water per SECOND. That's why they don't have any trouble with nitrogen build up.
So, I hope that water changes are a part of your tank regime.
With a little luck and more spare time, I'll do some research on the other components of the tank (buffer/pH chemistry, dissolved oxygen, TDS, etc) and come up with some more... but this is long enough already.

Last edited:
spot on ogre... epic even.
and these tags are hilarious... "1 vs 1000, arguments r us, deep sand beds, electroly, epic fail, i like it dirty!, living in a outhouse, magical thinking > wc, nitrates, no showers needed, water, water changes"
and these tags are hilarious... "1 vs 1000, arguments r us, deep sand beds, electroly, epic fail, i like it dirty!, living in a outhouse, magical thinking > wc, nitrates, no showers needed, water, water changes"
OgreMkV
Father of Earth's Next Emperor
pH and Aquariums – Why water changes are necessary…
The OP has requested scientific evidence for the process of water changes. My first article in this series covered the chemistry and the effects on fish of the nitrogen cycle. This article will cover pH, buffer chemistry, and the effects on fish.
First let’s cover why this is important. Here’s the some of the research I found on the effects of pH. This is only two and the most dramatic, but the others are out there. I found these in less than 15 minutes of semi-diligent googling (my boss kept asking me to do work).
Toxicity of pH
http://www.apms.org/japm/vol16/v16p40.pdf
Trent, L, et. al. 1978. Toxicity Of Sulfuric Acid To Aquatic Plants And Organisms
Journal of Aquatic Plant Management 16:40-43.
In this article, several species of plants and animals were subjected to (admittedly) quickly lowered pH levels. This was done by adding sulfuric acid to several varieties of aquariums and test jars with various species in them. The results are very dramatic.
Snails: pH 3 within 2 minutes, all snails dead. pH 5 severe mortality for the first two hours, then leveled off. pH 8.3 no mortality
Scud: pH 3 within 6 hours, all scud dead. pH 5 continuous mortality over first 24 hours, no mortality thereafter. pH 8.3 no mortality
FW Shrimp: pH 3 within 2 hours, all dead. pH 5 within 4 hours, 25% mortality, none thereafter.
Largemouth Bass: pH 3 within 24 hours all dead. pH 5 minor mortality. pH 8.3 no mortality
Mosquitofish: pH 3 within 8 hours, 60% mortality; within 24 hours 80% mortality. pH 5 within 4 hours, 20% mortality, none thereafter.
Yes, this was a rapid pH change. However, chemically speaking, such a rapid change is possible. Again though, just because the fish survived, that does not mean that they were healthy and happy. The article was really about killing weeds with sulfuric acid, not about the fish. I would love to see a study on the long term effects on health and longevity of fish exposed in this way…
Oh wait…
http://psasir.upm.edu.my/3664/1/Effects_of_Nitrite_and_pH_on_a_Tropical_Fish_Fry,_Puntius_gonionotus_(Bleeker).pdf
This is a paper in a Malaysian science journal so I won’t try to transcribe the actual reference, but the link will take you to it. The title is: Effects of Nitrite and pH on a Tropical Fish Fry, Puntius gonionotus (Bleeker)
NOTE: In this article LC50 is the same as LD50 that I described in the article on the nitrogen cycle.
I’ll just paste the abstract here:
The effects of short term and long term exposure of a tropical fish fry, Barbodes gonionotus (Bleeker), to pH and nitrite separately and in combination, were evaluated using static and flow-through bioassays respectively. The 96-hour LC50 values of pH and nitrate were 4.9 and 7.91 mg/l N02-N respectively. However, the 96-hour LC50 of pH was higher (5.4 pH unit) in the presence of nitrite 5.00 mg/l N02-N) than that without nitrite. At pH 5.00,100% mortality was found at 4.00 mg/l N02-N concentration after 48-hour exposure. Under long-term exposure, the growth rates of the fish fry decreased with increased nitrite concentrations. Fish fry grown at 2.00 I7lg/l N02-N had significantly lower growth rate (P < 0.05) than the control, but had a significantly higher rate (P < 0.05) than in the 4.00 mg/l N02-N (PH 7.33-7.56). One hundred percent mortality occurred within 30 days at pH 5.00 - 7.00 when the fish were exposed to 4.00 mg/l N02-N concentration at the same time. The study demonstrated that the effects of combined pH and nitrite on the survival and growth rates of the fish fry were more serious than the effects of each factor separately.
So a very meager nitrite level of 4 mg/l combined with a pH between 5 and 7 resulted in 100% death within 30 days. Yech.
Now as far as the chemistry of pH is concerned, it was, again, somewhat difficult to put all of into perspective because it’s so basic that everyone knows you have to watch the pH. I wanted to be able to explain it for everyone so I did some digging.
ACID OR BASE
First, some chemistry primers. Pure water has a neutral pH. The reason for this chemically is that pH is determined by the relative concentrations of the H+ (really H3O+, but H+ is close enough for us) ion and the OH- ion. If water has an excess of H+ ions then it is acidic. The more H+ ions there are, then the more acidic the water is. This is measured from 0-7 on the pH scale. If there is an excess of OH- ions, then the water is basic (or alkaline). Again, the more OH- present, then more basic the water is. This is measured from 7-14 on the pH scale. One more important bit of info here, the pH scale is logarithmic, that is a pH 6 has 10 times more H+ than a pH 7. A pH 5 has ten times more H+ than a pH 6.
OH- + H+ -> H20
Water, is always breaking apart and those ions are always coming back together. That’s OK as long as the amount of H+ and OH- are the same (or very close), then the water is pH 7.
Acids, when they dissolve in water, add extra H+ ions. Acids always have hydrogen in their chemical formula. H2SO4 for example, from the first article, is sulfuric acid. When you poor this in water, you get 2 H+ ions and a sulfate ion (SO4--). The H+ ions will cause the pH to decrease.
Bases, on the other hand, release OH- ions when they dissolve in water and their chemical formula always contains OH- (hydroxide). Sodium hydroxide (NaOH) for example. The OH- ions will cause the pH to increase.
The ideal pH range depends on the species kept, but for freshwater 6-7 is a common range and for saltwater 8.3 is ideal for reefs.
BUFFERING
Buffering is the ability of the water to resist changes in pH. The buffering capacity is measured in carbonate hardness (KH). As acids are added to the water column (urea, products from the nitrogen cycle, etc) the carbonates react with the acids to neutralize them and restore the chemical balance of the system.
One very important point to make here is that these are not one way chemical reactions. They are easily reversible and the direction of the reaction depends on the concentration of reactants and products. I’ll explain more in a minute.
(1) CO2 + H2O <> H2CO3
This more commonly read as carbon dioxide gas reacts with water to form carbonic acid. This is why the CO2 emissions of our power plants are destroying the oceans. If the CO2 increases, then the reaction goes from CO2 and water to carbonic acid (which lowers the pH of the ocean, dissolving snail shells, corals, shrimp, etc). If we remove a lot of the CO2 from the air, then the reaction will go the other way and the acid in the water will disassociate into CO2 and water.
As an aside: a guy I know has a large reef tank with digital pH gear. He can watch the pH drop when he has a large party at his house. All the guests are exhaling carbon dioxide and changing the equilibrium of the reaction. It causes more carbonic acid to form, lowering the pH of the tank.
(2) H2CO3 <> H+ + HCO3-
(3) HCO3- <> H+ + CO3- -
These two reactions show that carbonic acid disassociates into a hydrogen ion (acid) and bicarbonate and then that disassociates into another hydrogen ion and the carbonate ion.
(4) XCO3 <> X++ + CO3- -
This one shows why limestone (CaCO3) affects pH.
(5) XHCO3 <> X+ + HCO3-
The importance of all these reactions is that carbonate is the most common buffer used in aquariums (Sodium carbonate and sodium bicarbonate, for example). Aragonite (CaCO3) is another common buffer in marine tanks and makes up the sand and/or liverock in the tank. However, it is also used in the shells of snails and corals.
The take away message here is that if you are depending on aragonite sand or liverock (or limestone for that matter) as a buffer in your tank, then you are also using the shells of snails and corals as your buffer.
You can get significant buffering out to a 100:1 ratio, so most buffers work over a range of 4 pH units (+2 and -2 from normal). For example, if you want to keep the pH the same, but your KH halves, then you have to halve the CO2 to keep the same ration and pH stable.
Obviously KH is a huge factor in our tanks (even the FW tanks to some degree).
HARDNESS
The general hardness (as opposed to carbonate hardness (above)) of the water refers to the combination of magnesium and calcium ions in the water (Mg+ and Ca+). The hardness interacts with the pH and KH such that changes in any of these will result in changes to the others.
Obviously, the Ca+ level is vitally important in marine aquariums as a source of the shells for corals. However, it is also important for FW fish and plants so that they achieve a proper osmotic balance.
This is why one of the best possible buffer is calcium bicarbonate.
Hard water is generally higher in pH and soft water generally lower in pH.
Now, here’s the money part. Why is this important for water changes?
The ability to control and maintain a proper pH in your tank is dependant upon the hardness (higher Ca+ and Mg+) or softness (lower Ca+ or Mg+) of the water.
Now if you have to lower the pH in a FW tank with hard water, then you’ll have serious problems. Based on the chemistry above, you can add acids to the water and the minerals will react with the acids forming carbonates and (in some cases) precipitating out of the water. You must first remove the minerals from the water before trying to change the pH. Alternately (note here) adding hardness to the water can drive pH higher and no amount of pH adjustment can help until the minerals are removed.
Similarly, if you have to raise the pH of a tank that has very soft water, you won’t be able to get it higher and keep it there. There are no minerals in the water to act as a buffer and keep the water pH higher. (Especially common in marine tanks… ask me how I know.)
Now, we can add chemical buffers… up to a point. Unfortunately, these chemicals build up in the aquarium over time. Then you reach a point where you can’t add anymore buffer to have an effect. At that point, you can have a major crash. A huge swing in pH, hardness, carbonates, all of the above, which will change your tank in hours from a perfect habit to that pH 3-5 I mentioned above.
In a soft water, low alkalinity (KH, buffering capacity) system, pH tends to decline. The metabolism of the nitrifying bacteria produces acids and these can reduce pH.
The pH is more stable in higher KH water, so pH should not be used as a factor for determining the water change schedule in that case, because toxins will build up to high levels in the water long before pH begins to drop. If you wait too long and the pH drops, it will drop fast and may kill many fish.
A water change in this instance can do several things.
This also shows us one myth: “Live rock and aragonite sand alone are not a good buffer for a marine aquarium.”
As an aside for the marine reefers. It's best to not use the 8.3 pH buffer and instead use the buffer that's 9.0 or better... just use a smaller amount. Both products contain the same chemicals. But the 8.3 adds a n acid buffer to reduce the pH (makes the water more acidic). However, the basic chemicals get used up maintaining the pH at 8.3 and then when they are all chemically removed from the system, the acidic buffer is the only one left and you get a sudden drop in pH.
This isn't science, just basic (ha ha) chemistry.
The OP has requested scientific evidence for the process of water changes. My first article in this series covered the chemistry and the effects on fish of the nitrogen cycle. This article will cover pH, buffer chemistry, and the effects on fish.
First let’s cover why this is important. Here’s the some of the research I found on the effects of pH. This is only two and the most dramatic, but the others are out there. I found these in less than 15 minutes of semi-diligent googling (my boss kept asking me to do work).
Toxicity of pH
http://www.apms.org/japm/vol16/v16p40.pdf
Trent, L, et. al. 1978. Toxicity Of Sulfuric Acid To Aquatic Plants And Organisms
Journal of Aquatic Plant Management 16:40-43.
In this article, several species of plants and animals were subjected to (admittedly) quickly lowered pH levels. This was done by adding sulfuric acid to several varieties of aquariums and test jars with various species in them. The results are very dramatic.
Snails: pH 3 within 2 minutes, all snails dead. pH 5 severe mortality for the first two hours, then leveled off. pH 8.3 no mortality
Scud: pH 3 within 6 hours, all scud dead. pH 5 continuous mortality over first 24 hours, no mortality thereafter. pH 8.3 no mortality
FW Shrimp: pH 3 within 2 hours, all dead. pH 5 within 4 hours, 25% mortality, none thereafter.
Largemouth Bass: pH 3 within 24 hours all dead. pH 5 minor mortality. pH 8.3 no mortality
Mosquitofish: pH 3 within 8 hours, 60% mortality; within 24 hours 80% mortality. pH 5 within 4 hours, 20% mortality, none thereafter.
Yes, this was a rapid pH change. However, chemically speaking, such a rapid change is possible. Again though, just because the fish survived, that does not mean that they were healthy and happy. The article was really about killing weeds with sulfuric acid, not about the fish. I would love to see a study on the long term effects on health and longevity of fish exposed in this way…
Oh wait…
http://psasir.upm.edu.my/3664/1/Effects_of_Nitrite_and_pH_on_a_Tropical_Fish_Fry,_Puntius_gonionotus_(Bleeker).pdf
This is a paper in a Malaysian science journal so I won’t try to transcribe the actual reference, but the link will take you to it. The title is: Effects of Nitrite and pH on a Tropical Fish Fry, Puntius gonionotus (Bleeker)
NOTE: In this article LC50 is the same as LD50 that I described in the article on the nitrogen cycle.
I’ll just paste the abstract here:
The effects of short term and long term exposure of a tropical fish fry, Barbodes gonionotus (Bleeker), to pH and nitrite separately and in combination, were evaluated using static and flow-through bioassays respectively. The 96-hour LC50 values of pH and nitrate were 4.9 and 7.91 mg/l N02-N respectively. However, the 96-hour LC50 of pH was higher (5.4 pH unit) in the presence of nitrite 5.00 mg/l N02-N) than that without nitrite. At pH 5.00,100% mortality was found at 4.00 mg/l N02-N concentration after 48-hour exposure. Under long-term exposure, the growth rates of the fish fry decreased with increased nitrite concentrations. Fish fry grown at 2.00 I7lg/l N02-N had significantly lower growth rate (P < 0.05) than the control, but had a significantly higher rate (P < 0.05) than in the 4.00 mg/l N02-N (PH 7.33-7.56). One hundred percent mortality occurred within 30 days at pH 5.00 - 7.00 when the fish were exposed to 4.00 mg/l N02-N concentration at the same time. The study demonstrated that the effects of combined pH and nitrite on the survival and growth rates of the fish fry were more serious than the effects of each factor separately.
So a very meager nitrite level of 4 mg/l combined with a pH between 5 and 7 resulted in 100% death within 30 days. Yech.
Now as far as the chemistry of pH is concerned, it was, again, somewhat difficult to put all of into perspective because it’s so basic that everyone knows you have to watch the pH. I wanted to be able to explain it for everyone so I did some digging.
ACID OR BASE
First, some chemistry primers. Pure water has a neutral pH. The reason for this chemically is that pH is determined by the relative concentrations of the H+ (really H3O+, but H+ is close enough for us) ion and the OH- ion. If water has an excess of H+ ions then it is acidic. The more H+ ions there are, then the more acidic the water is. This is measured from 0-7 on the pH scale. If there is an excess of OH- ions, then the water is basic (or alkaline). Again, the more OH- present, then more basic the water is. This is measured from 7-14 on the pH scale. One more important bit of info here, the pH scale is logarithmic, that is a pH 6 has 10 times more H+ than a pH 7. A pH 5 has ten times more H+ than a pH 6.
OH- + H+ -> H20
Water, is always breaking apart and those ions are always coming back together. That’s OK as long as the amount of H+ and OH- are the same (or very close), then the water is pH 7.
Acids, when they dissolve in water, add extra H+ ions. Acids always have hydrogen in their chemical formula. H2SO4 for example, from the first article, is sulfuric acid. When you poor this in water, you get 2 H+ ions and a sulfate ion (SO4--). The H+ ions will cause the pH to decrease.
Bases, on the other hand, release OH- ions when they dissolve in water and their chemical formula always contains OH- (hydroxide). Sodium hydroxide (NaOH) for example. The OH- ions will cause the pH to increase.
The ideal pH range depends on the species kept, but for freshwater 6-7 is a common range and for saltwater 8.3 is ideal for reefs.
BUFFERING
Buffering is the ability of the water to resist changes in pH. The buffering capacity is measured in carbonate hardness (KH). As acids are added to the water column (urea, products from the nitrogen cycle, etc) the carbonates react with the acids to neutralize them and restore the chemical balance of the system.
One very important point to make here is that these are not one way chemical reactions. They are easily reversible and the direction of the reaction depends on the concentration of reactants and products. I’ll explain more in a minute.
(1) CO2 + H2O <> H2CO3
This more commonly read as carbon dioxide gas reacts with water to form carbonic acid. This is why the CO2 emissions of our power plants are destroying the oceans. If the CO2 increases, then the reaction goes from CO2 and water to carbonic acid (which lowers the pH of the ocean, dissolving snail shells, corals, shrimp, etc). If we remove a lot of the CO2 from the air, then the reaction will go the other way and the acid in the water will disassociate into CO2 and water.
As an aside: a guy I know has a large reef tank with digital pH gear. He can watch the pH drop when he has a large party at his house. All the guests are exhaling carbon dioxide and changing the equilibrium of the reaction. It causes more carbonic acid to form, lowering the pH of the tank.
(2) H2CO3 <> H+ + HCO3-
(3) HCO3- <> H+ + CO3- -
These two reactions show that carbonic acid disassociates into a hydrogen ion (acid) and bicarbonate and then that disassociates into another hydrogen ion and the carbonate ion.
(4) XCO3 <> X++ + CO3- -
This one shows why limestone (CaCO3) affects pH.
(5) XHCO3 <> X+ + HCO3-
The importance of all these reactions is that carbonate is the most common buffer used in aquariums (Sodium carbonate and sodium bicarbonate, for example). Aragonite (CaCO3) is another common buffer in marine tanks and makes up the sand and/or liverock in the tank. However, it is also used in the shells of snails and corals.
The take away message here is that if you are depending on aragonite sand or liverock (or limestone for that matter) as a buffer in your tank, then you are also using the shells of snails and corals as your buffer.
You can get significant buffering out to a 100:1 ratio, so most buffers work over a range of 4 pH units (+2 and -2 from normal). For example, if you want to keep the pH the same, but your KH halves, then you have to halve the CO2 to keep the same ration and pH stable.
Obviously KH is a huge factor in our tanks (even the FW tanks to some degree).
HARDNESS
The general hardness (as opposed to carbonate hardness (above)) of the water refers to the combination of magnesium and calcium ions in the water (Mg+ and Ca+). The hardness interacts with the pH and KH such that changes in any of these will result in changes to the others.
Obviously, the Ca+ level is vitally important in marine aquariums as a source of the shells for corals. However, it is also important for FW fish and plants so that they achieve a proper osmotic balance.
This is why one of the best possible buffer is calcium bicarbonate.
Hard water is generally higher in pH and soft water generally lower in pH.
Now, here’s the money part. Why is this important for water changes?
The ability to control and maintain a proper pH in your tank is dependant upon the hardness (higher Ca+ and Mg+) or softness (lower Ca+ or Mg+) of the water.
Now if you have to lower the pH in a FW tank with hard water, then you’ll have serious problems. Based on the chemistry above, you can add acids to the water and the minerals will react with the acids forming carbonates and (in some cases) precipitating out of the water. You must first remove the minerals from the water before trying to change the pH. Alternately (note here) adding hardness to the water can drive pH higher and no amount of pH adjustment can help until the minerals are removed.
Similarly, if you have to raise the pH of a tank that has very soft water, you won’t be able to get it higher and keep it there. There are no minerals in the water to act as a buffer and keep the water pH higher. (Especially common in marine tanks… ask me how I know.)
Now, we can add chemical buffers… up to a point. Unfortunately, these chemicals build up in the aquarium over time. Then you reach a point where you can’t add anymore buffer to have an effect. At that point, you can have a major crash. A huge swing in pH, hardness, carbonates, all of the above, which will change your tank in hours from a perfect habit to that pH 3-5 I mentioned above.
In a soft water, low alkalinity (KH, buffering capacity) system, pH tends to decline. The metabolism of the nitrifying bacteria produces acids and these can reduce pH.
The pH is more stable in higher KH water, so pH should not be used as a factor for determining the water change schedule in that case, because toxins will build up to high levels in the water long before pH begins to drop. If you wait too long and the pH drops, it will drop fast and may kill many fish.
A water change in this instance can do several things.
- It can remove or add minerals
- It can make your tank water harder or softer
- It increase the stability of your tank by 'reseting' part of the chemical buffer system.
This also shows us one myth: “Live rock and aragonite sand alone are not a good buffer for a marine aquarium.”
As an aside for the marine reefers. It's best to not use the 8.3 pH buffer and instead use the buffer that's 9.0 or better... just use a smaller amount. Both products contain the same chemicals. But the 8.3 adds a n acid buffer to reduce the pH (makes the water more acidic). However, the basic chemicals get used up maintaining the pH at 8.3 and then when they are all chemically removed from the system, the acidic buffer is the only one left and you get a sudden drop in pH.
This isn't science, just basic (ha ha) chemistry.
spot on ogre... epic even.
and these tags are hilarious... "1 vs 1000, arguments r us, deep sand beds, electroly, epic fail, i like it dirty!, living in a outhouse, magical thinking > wc, nitrates, no showers needed, water, water changes"
:lol::lol::lol:
- Status
- Not open for further replies.



