Just to confirm:
"add another full ammonia dose (Dose #4) and then test in 24 hours.
If ammonia and nitrite both read 0 ppm, you are cycled. Do a large water change, be sure the water is the proper temperature, and add fish. The odds are this will not be the case quite this soon.
If ammonia and nitrite do not both read zero, continue to test daily. Whenever ammonia is again at .25 ppm or less and nitrite is clearly under 1 ppm, add the full amount of ammonia (Dose #5) and test in 24 hours. "
The timing for the cycling method in the article I linked is based on a from the start process. Where you are was somewhere in the middle and a bit messed up. So the exact timing to get to the desired 0/0 is somewhat variable. The goal is not to need another water change until the cycle is complete. Unless things go wonky again for an unexpected reason, your tank should get back on track within a matter of days.
Test tomorrow morning and let's see where your readings stand. My expectation is that ammonia should drop and that nitrite should rise. I would expect ammonia is clearly down from 3.25 ppm after this morning's addition should have made it, and that nitrite is clearly up from wherever it was this morning. Nitrite should not hit 5 ppm. However, if it does, I am not worried as It cannot reach cycle stalling level based on how much ammonia was added and how many nitrite oxidizers there already are.
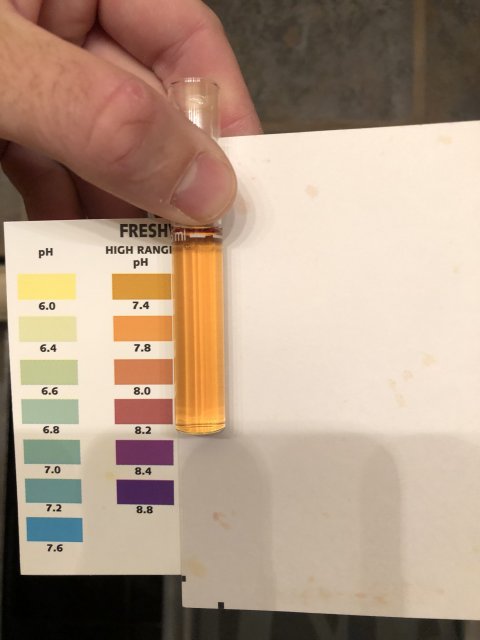
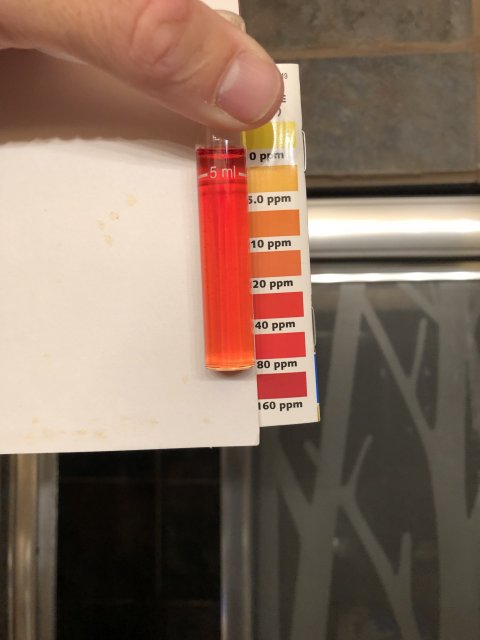
numbers don’t seem to have changed much this morning. 2-3 ammonia, I’d say 1 on Nitrite but looked darker, and around 20 nitrate. Ph is 7.5 range