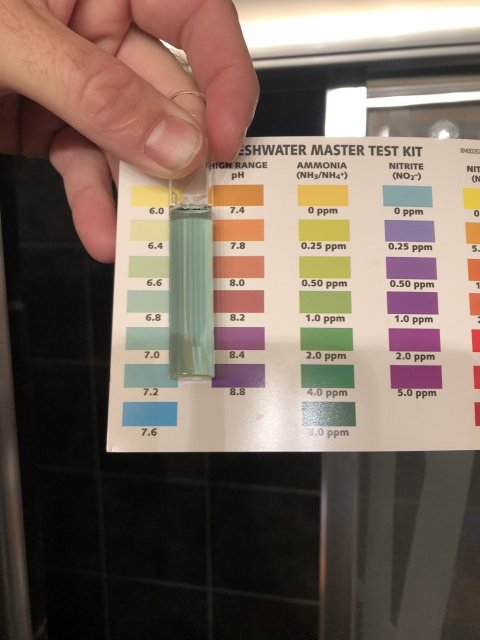
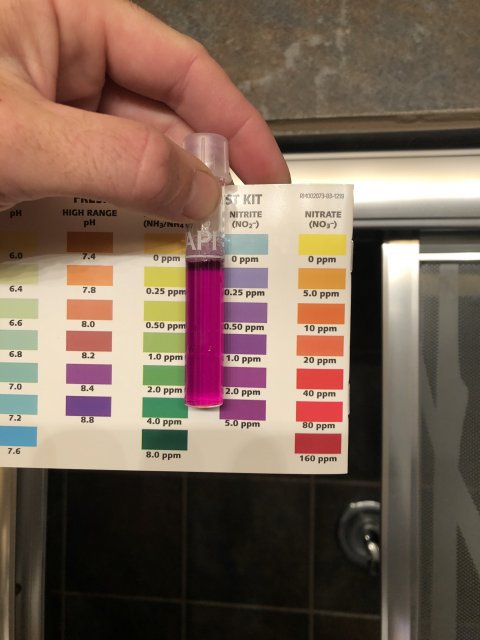
Every time you post pictures of your test result I keep asking myself why the colors in the test tube never seen to come close to those on the card.This is most true for your nitrite and ammonia results. I hate to ask you to do this, but can you do the following tests?
- Use distilled water only and test it for ammonia, nitrite and nitrate. This will let us know that the tests are working properly. (All 0s are expected.)
- Then do the same for your tap water (do not add dechlor). This will let us know if ammonia, nitrite or nitrate are coming in with your tap. Hopefully, you get all 0s here. However, if you do get any readings above 0, this might point to what is causing things to be happening as they are.
Where we are now, from what I see, is the pH is coming down and that is not good in terms of keeping the ammonia bacteria "happy." There are a variety of nitrifying bacteria. What makes them different is the concentration of ammonia on which they can thrive (as well as salinity). The recently discovered Archaea can thrive only in the lowwst ammonia concentrations. The can thrive in levels too low to support the bacteria in our tanks, On the other hand too much ammonia will result in bacteria that do not thrive in tank levels of ammonia but are stars in waste water treatment because of how much ammonia they need and can handle.
Since I was not involved in your initial cycling, I am not certain of how much ammonia you may have actually been adding. If it was too much, you may have developed the "wrong" strain of ammonia bacteria. If so, when we did the reset what had to happened was for the wrong bacteria to die back and the desired bacterial strains had to start almost from scratch. However, that does not explain why the colors I see in your pictures are so far from what is on the card.
While things are not going as rapidly as I expected, they are still clearly going in the right direction. So, after you do the two sets of test above, we will reset the tank again. Do as big a water change as possible. Do not add dechlor until after you do all of the the following. Please record all the test results above and below.
1. Test the tank water for ammonia, nitrite and nitrate.
2. Add a full dose of ammonia. Here it may get tricky. It is important not to add any more ammonium chloride than it takes to get the tank to 3 ppm. Because we are using powder to create ammonia, it is difficult to do accurate fractional changes. So you need to do something similar to the diluted testing for nitrite.
If the ammonia reading in #1 above is .25 ppm or less, just do the normal full 1/4 teaspoon dose. However, if the ammonia is above .25 ppm, you will have yo add less than 1.4 teaspoon. Here is the easiest way to do that. Divide 3 by the ammonia reading in #1. For example of you test .75 ppm then .75/3 = .25 or 25% aka 1/4. So you will only need 3/4 of the full dose. So, put 4 ounces of distilled water into your measuring cup and add the 1/4 teaspoon of ammonium to that and mix it. Then add 3 ounce of this to the tank. Wait about 15 minutes and test for ammonia. This way we will know if the dosing of ammonia is accurate. Does 1/4 teaspoon actually produce very close to 3 ppm?
Then add the dechlor. You can change the volume of distilled to make it easier to achieve the fractional dosing. For example. If you test and get .50 ppm, that is 1/6 of 3 ppm. So use 6 ounces of distilled and then only add 5 to the tank, etc. You may already already realize how to do these things, but I try to overly detail the math and methods so that anybody reading along will understand how to do this stuff.
The nice thing about chloramine is that the presence of ammonia in the water keeps the bacteria alive. The chloramine can put them to sleep but the ammonia counteracts that. This works great for the lower levels of residual chloramine normally still in tap water. So you need not worry, the bacteria you have will not be harmed. The exposure to any residual chloramine will not harm them in the short time frame involved. What I want to avoid is for the ammonia part of chloramine to be liberated by the dechlor and thus showing up in the test.
I know all of this can be frustrating. But it is kind of like an experiment. The difference is we are working with a few uncontrolled variables here. Firstly, what may be in one's tap water is always a bit of a mystery, even when we have water reports. Secondly, things can and do change. Thirdly, even if they are dead accurate, there is so much stuff involved that it can be impossible to know what effect they have on testing or cycling. Add to that the fact that it is almost impossible to know how much of the nitrifying bacteria we have in a tank at the outset. The less we start with, the longer the cycle takes.
Every tank is unique to some extent. Add to that the vagaries of the test kits and the fact that we are measuring ammonium with teaspoons and measuring cups and it should be easy to see how many places it is possible for things to be off a bit in either direction. The one thing that we have to supply is the patience needed and the understanding of the process involved. Finally, add in the remote component and things get more complicated.
That said, do not despair. We will get you cycled. We know you are generally moving in the right direction. Things would work much easier if you only lived next door to me. Then I could see what you see and we could do things more quickly and thus more efficiently. Look at the bright side. When you set up your next tank, you will know so much more and things should go that much faster.