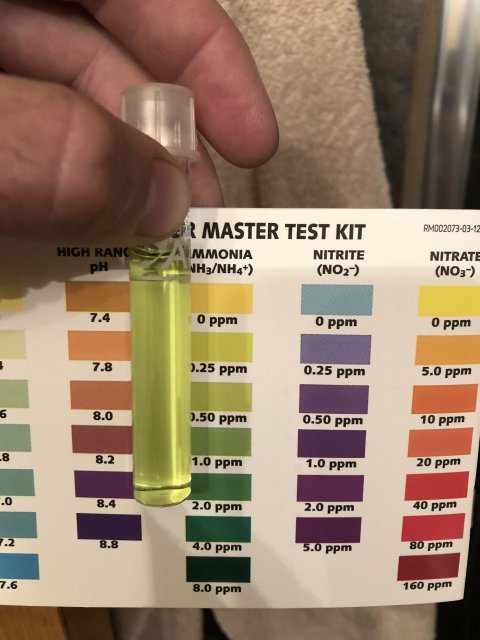
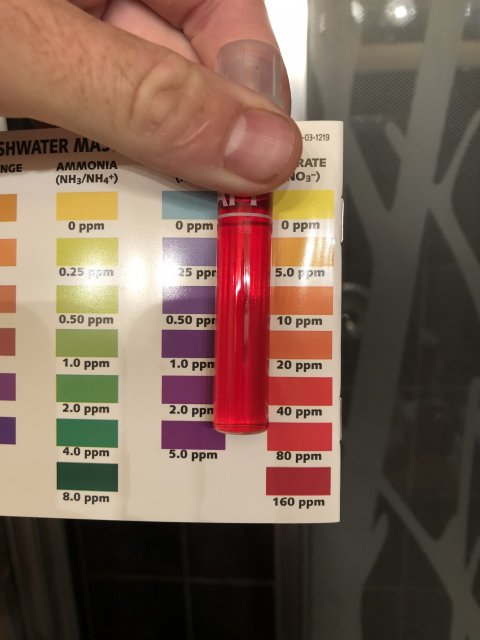
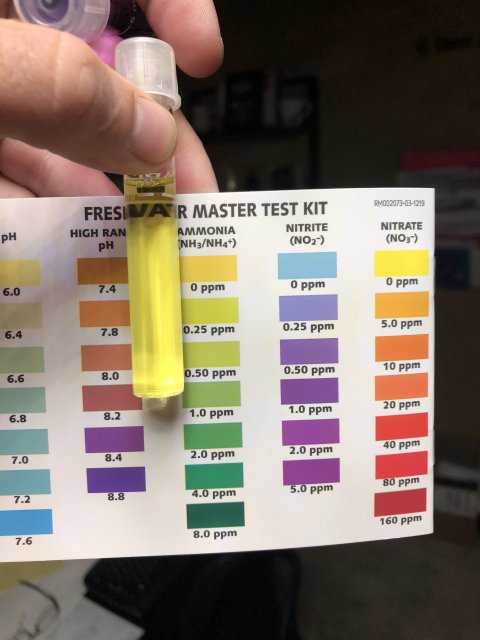
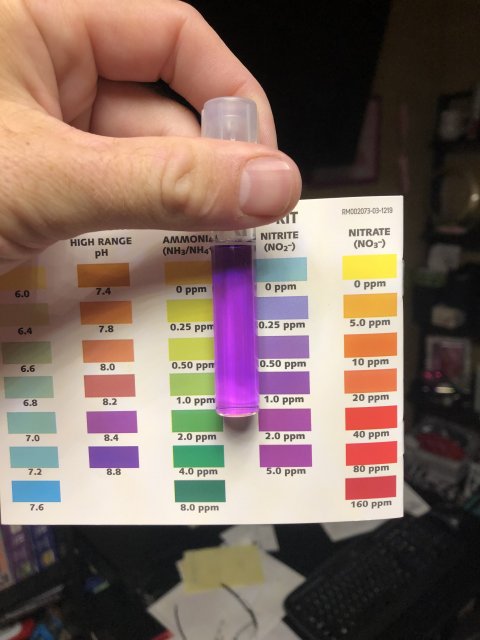
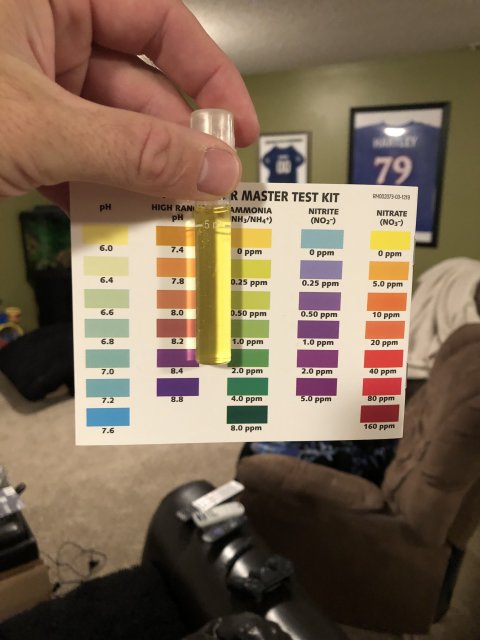
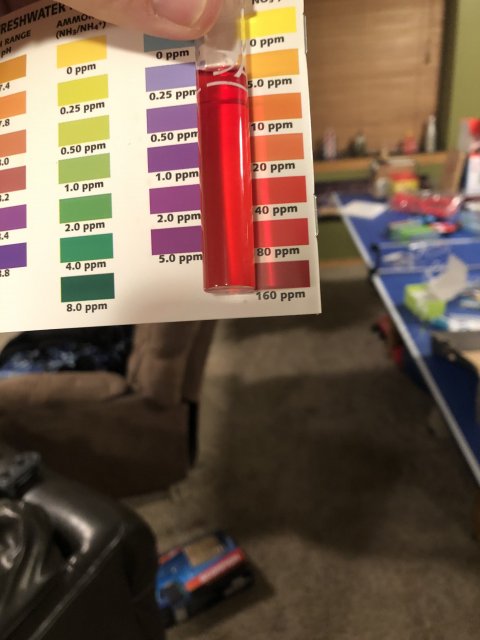
Ammonia is super, nitrite I cannot tell from the pic as usual. I see no match to any color at all.
Just a quick observation. When you take the picture whatever device you are using and whatever light is illuminating the tube and card will affect the color one might see. Anybody who views the picture has a monitor that determines how colors appear. Professional photographers have software you would not believe for calibrating their screens to produce "true" colors. And then there is the room lighting where the screen is being viewed to consider.
Finally, everybody has their own unique eyes. Color perception is subjective since it is not only the bio-physics of our optuical system at work, perception is how our unique brains translate the information. So you can email/text 10 fish keepers your picture to them all in an email and ask them to reply as to what color they see and you will gent many different answers.
This is why accurate colorimetric tests are not read by humans, they are measured spectroscopically using expensive equipment to determine to the exact nanometer wavelength to determine what the color is. I tried to get Commander Data to beam down and check your test results, but unfortunately he was otherwise occupied in another galaxy. ??
In any case, when you know nitrite is clearly under 1 ppm. Dose a short 1/4 teaspoon again. (By short I mean not quite level, I want a bit less than 3 ppm but not by much.) Please check for either nitrate or KH first. If you are close to the 160 or above for Nitrate or at 2 dg/35 ppm for KH, do a 50% water change before dosing.
I can offer one idea here if you want to reshoot the nitrate picture. It appears to me that you have too much light where you are taking the picture. I think you may be washing out something that changes how things look, especially in the tube. Is there a way you can shoot in a somewhat less bright light? If you are using artificial light, move farther from the light. If you are using sunlight, make sure the sun is behind you, but shoot standing in the shade or inside with the sun coming in a window. That way you can move further from the sunshine/window to make things a bit less bright. I could be wrong here, but it is worth a try if you want.