In theory that will work. In practice you are working against the clock. A heavily planted tank will arguably deplete CO2 faster than simple surface agitation will replenish. Otherwise injecting CO2 wouldn't be benefical and a simple airstone would do the trick. The ph will swing from photo period to non-photo period - and that is from less dissolved CO2 during photo period. My theory is that the balancing act to equalize O2 and CO2 levels to atmospheric levels does not happen fast enough - if it did there is no reason for the ph swing. That little airpump for aquarium use is barely strong enough to fight against the water pressure - bubbles rise straight up and leave. I still don't think that is fast enough or rather not a big enough total area.
My aquarist rant
- Thread starter wesleydnunder
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Threads like these are why I absolutely love this website. I know nothing of c02, and am thus useless for this topic.
$0.02 as always.
$0.02 as always.
That's not very realistic, given the low density of CO2 in the atmosphere, and the reason bubbles don't work--it's mostly nitrogen and oxygen, and the nitrogen isn't even in a form the plants use.
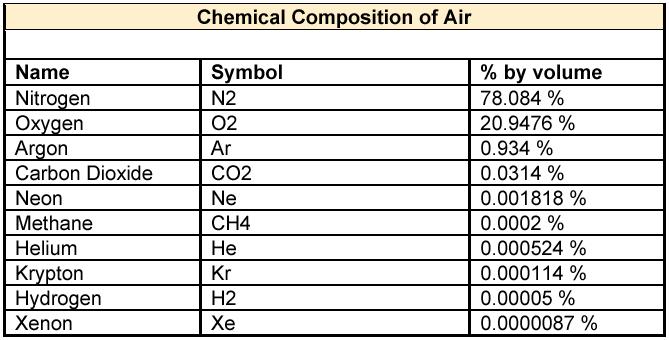

That's not very realistic, given the low density of CO2 in the atmosphere, and the reason bubbles don't work--it's mostly nitrogen and oxygen, and the nitrogen isn't even in a form the plants use.

...and bubbling from the air will only dissolve a few ppm of co2 into the water as a result of it's low percentage in the air, while dissolved oxygen in the water will get to around 7.5 ppm at 30 C. It's only when we add pure co2 to the water that the dissolved percentage rises above the natural value.
Mark
Let's not get ahead of ourselves until there is also a table how the composition translates into each gases concentration in water when water and air is in equilibrium. That is not at all apparent from the composition of air alone.That's not very realistic, given the low density of CO2 in the atmosphere, and the reason bubbles don't work--it's mostly nitrogen and oxygen, and the nitrogen isn't even in a form the plants use.

We probably need a chemist for that though. At least I am unable to find it.
CO2 in water is highly variable, but it quickly gases off under agitation. End result, CO2 levels in water are much lower than in air. Bubbling air through the water won't increase it because the level of CO2 in the air is too low. Kind of like pouring water into a glass that's already full of water...no net change in the water level. This explains a bit about it in the context of natural bodies of water and the impact on pH: http://www.fondriest.com/environmental-measurements/parameters/water-quality/ph/
It is factually wrong to say that CO2 only gases off with agitation. If the level of dissolved CO2 is too low, then agitation will add CO2 to the water. Same with O2 - if there is too much oxygen dissolved in water, then agitation will gas off that oxygen. Agitation is adding oxygen to water when there is not enough oxygen in it. Are those numbers in % under water low? Yes they are, I am not arguing that. CO2 in air is "only" 0.0314% (your data) - statistically insignificant, yes? Yet it is plenty to make trees and other plants grow.
It is factually wrong to say that CO2 only gases off with agitation. If the level of dissolved CO2 is too low, then agitation will add CO2 to the water. Same with O2 - if there is too much oxygen dissolved in water, then agitation will gas off that oxygen. Agitation is adding oxygen to water when there is not enough oxygen in it. Are those numbers in % under water low? Yes they are, I am not arguing that. CO2 in air is "only" 0.0314% (your data) - statistically insignificant, yes? Yet it is plenty to make trees and other plants grow.
Look more closely at the post. It doesn'y suggest that co2 ONLY gasses off under agitation, but that it gasses off QUICKLY under agitation.
Mark
Makes no difference that the process of gasing off is faster than dissolving CO2 into water. Depending on the level it will either do one or the other (on average). Speed only matters insofar that the supply of a nutrient (e.g. CO2) needs to be faster than it is used up by a plant. Simple matter of supply and demand.Look more closely at the post. It doesn'y suggest that co2 ONLY gasses off under agitation, but that it gasses off QUICKLY under agitation.
Mark
If you know for a fact that agitation with air will never be able to supply CO2 fast enough for a planted high light aquarium, then please quote a scientific study saying so. Otherwise you are simply using a "fox terrier" argument of: "a high light planted aquarium must have CO2 injection of some sort to raise CO2 levels unnaturally high." Sure it will work, but doesn't necessarily mean it is the only way.
Do you have any evidence that agitation adds CO2? I've not seen anything to indicate as much, since there is not level of CO2 that is standard in water and what DOES occur is usually found in conjunction with an underwater source, such as an algae bloom.
Asking for 'scientific studies' on a subject that isn't studied in lab conditions is specious. If you'd like more information, rather than just splashing aspersion here, go read the Barr Reports.
Asking for 'scientific studies' on a subject that isn't studied in lab conditions is specious. If you'd like more information, rather than just splashing aspersion here, go read the Barr Reports.


